Iec 60601 : Ee Overview Of Iec 60601 1 Scope And Normative References
There are two modes of operation described in IEC 60601-1 edition 31. General requirements tests and guidance for alarm systems in medical electrical equipment and medical electrical systems.

Iec 60601 1 2005 Amd2 2020 Iec Normen Vde Verlag
BSRAAMIIEC 60601-2-21-202x Medical Electrical Equipment - Part 2-21.

Iec 60601. ALL CAPITAL LETTERS identifies a defined term for the IEC 60601 series of standards within this blog. When a device is classified as non-CONTINUOUS OPERATION there is some type of. IEC 60601-1 Compliance Documents to evaluate medical electrical equipment to the applicable standards.
IEC 60601-2-412009 applies to the basic safety and essential performance of surgical luminaires and luminaires for diagnosis. IEC 60601-12021 SER Series Medical electrical equipment - ALL PARTS. Electromagnetic Disturbances - Requirements And Tests.
General requirements for radiation protection in diagnostic X-ray equipment. 1 CONTINUOUS OPERATION and 2 non-CONTINUOUS OPERATIONS. IEC 60601-1 has undergone a number of significant revisions over the years in an effort to remain current with new and advanced medical technologies.
Iec 60601-1-82006 Medical electrical equipment Part 1-8. It amends and supplements IEC 60601-1 third Edition 2005 hereinafter referred to as the general standard. As a Nationally Recognized Testing Laboratory NRTL approved by OHSA Intertek is an industry leader providing Testing Certification and in-lab support to help you navigate the requirements of the IEC 60601 series of standards.
10 b1994 Medical electrical equipment - Part 1. General Requirements For Basic Safety And Essential Performance - Collateral Standard. IEC 60601-12005 contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment.
From a global perspective every major medical device market of the world expects testing of your end product to IEC 60601. IEC 60601 is a series of technical standards for the safety and effectiveness of medical electrical equipment. General requirements for basic safety and essential performance Collateral standard.
The latest set of changes was introduced with the 2012 publication of Amendment 1 to IEC 60601-1. For purposes of medical device testing IEC 60601 is one of hundreds of family standards the IEC has developed for safety testing different types of products. General requirements for basic safety and essential performance.
The IEC 60601-1 Standard itself states ThisStandard applies toMEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMSreferred to as ME EQUIPMENT and ME SYSTEMS Note. For certain types of medical electrical equipment these requirements are either supplemented or modified by the special requirements of a collateral or particular standard. IEC 60601-1-112015 applies to the basic safety and essential performance of medical electrical equipment and medical electrical systems for use in the home healthcare environment.
The Primary Standard The primary standard governing medical device design is formally known as IEC 60601-1 - Medical electrical equipment - Part 1. IEC 60601-1-2 Edition 40 2014-02 Medical Electrical Equipment - Part 1-2. This second edition cancels and.
Particular Requirements for the Basic Safety and Essential Performance of Infant Radiant Warmers identical national adoption of IEC 60601-2-21 and revision of ANSIAAMIIEC 60601-2-21-2009 R2014 Stakeholders. General requirements for safety - 3. ENIEC 62368-1 Product Safety Standard Will Replace ENIEC 60950-1 and ENIEC 60065 as of December 2020 ENIEC 62368-1 is a product safety standard replacing ENIEC 60950-1 Information Technology Equipment and Safety and ENIEC 60065 Audio Video and similar Electronic Apparatus Safety requirements.
This seminar provides an overview of the IEC 60601. It applies regardless of whether the medical electrical equipment or medical electrical system is intended for use by a lay operator or by trained healthcare personnel. The main IEC 60601-1 standard referred to in Europe as EN 60601-1 and in Canada as CSA 60601-1 is an umbrella for numerous subsidiary standards variously known as collateral or particular standards.
Please contact us with any questions. With our team of experts globally Intertek is positioned to help determine standard applicability and execution.
Iec 60601 1 Medical Electrical Equipment Wo Tuv Rheinland
Iec 60601 Medical Design Standards For Power Supplies Plianced Inc
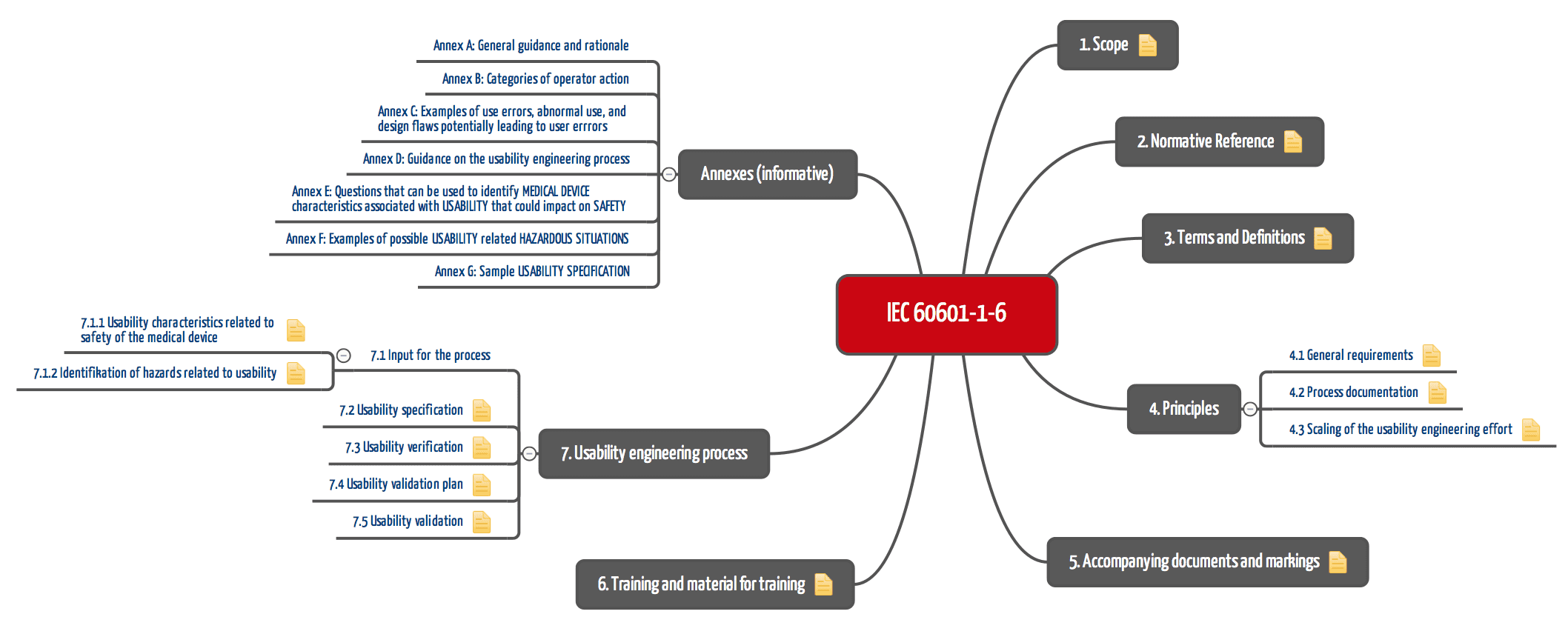
Iec 60601 1 6 Die Erganzungsnorm Zur Gebrauchstauglichkeit
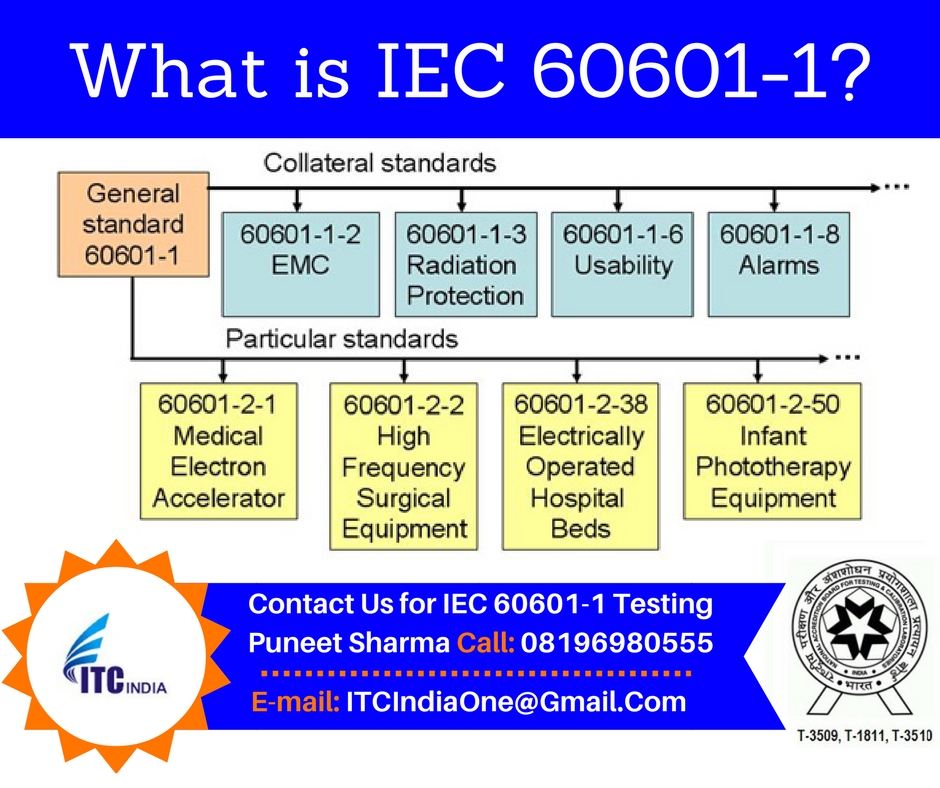
What Is Iec 60601 1 Electrical Safety Testing Lab

Iec 60601 1 Medical Power Supplies Cui Inc
Iec 60601 Medical Electrical Equipment Classification Faqs Medical Device Academy Medical Device Academy

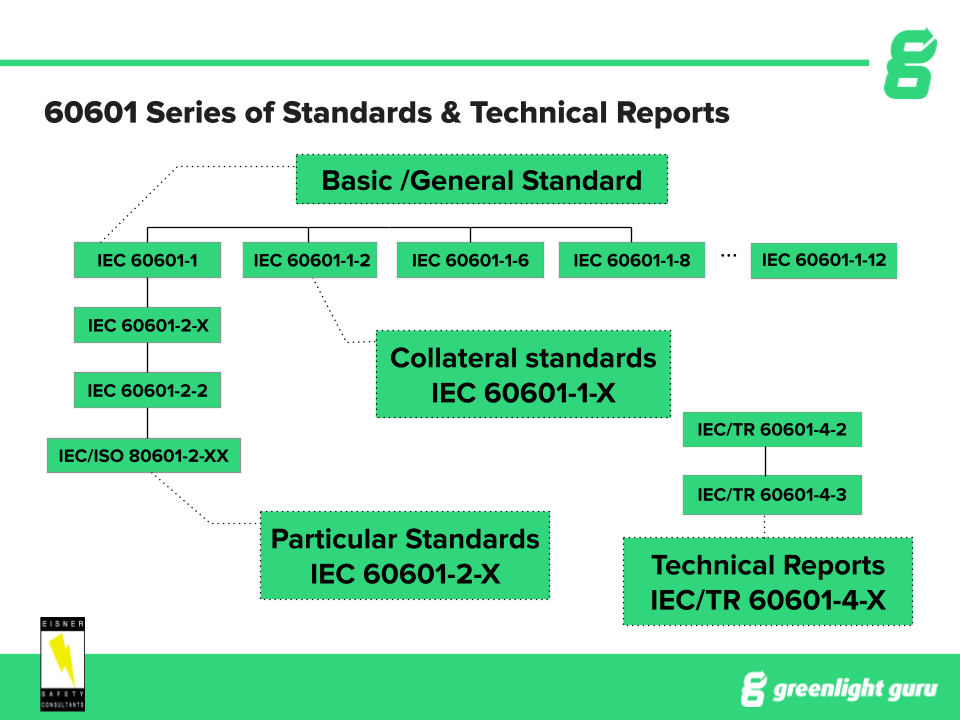
60601 1 Edition 3 1 And Understanding Iec60601 1 Document Structure Globtek

Iec 60601 1 Evolution Of Electrical Safety Standards To Match Device Development By Parul Chansoria Medium
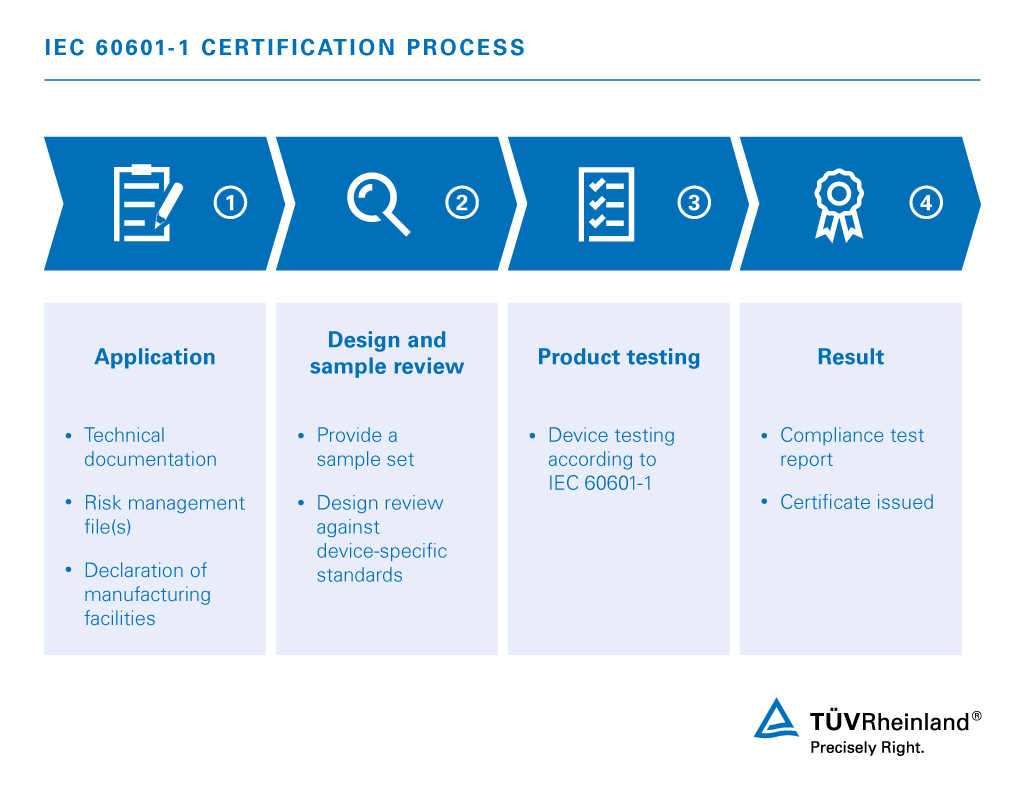
15 Steps To Getting Approval For Iec 60601 1

Iec 60601 1 12 2014 Iec Normen Vde Verlag
Ee Overview Of Iec 60601 1 Scope And Normative References
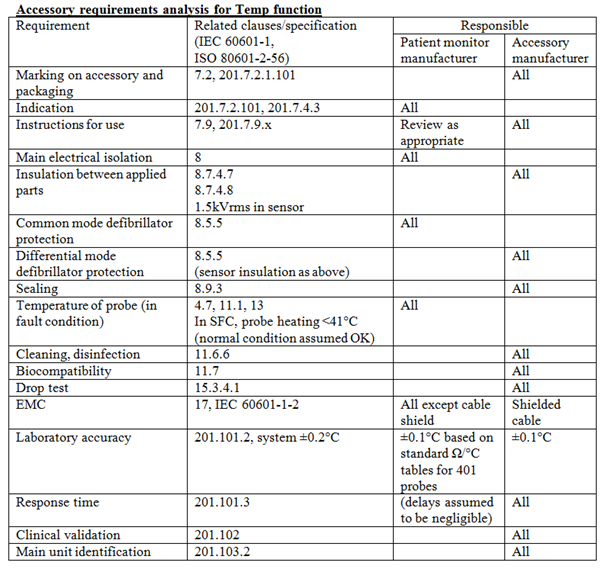
Iec 60601 1 And Accessories Medteq

Benefits Of Using Iec 60601 Compliant Components In Medical Devices
Medical Emc Iec 60601 1 2 2014 Edition 4 Northwest Emc

Iec 60601 1 8 2006 Amd2 2020 Amendment 2 Medical Electrical Equipment Part 1 8 General
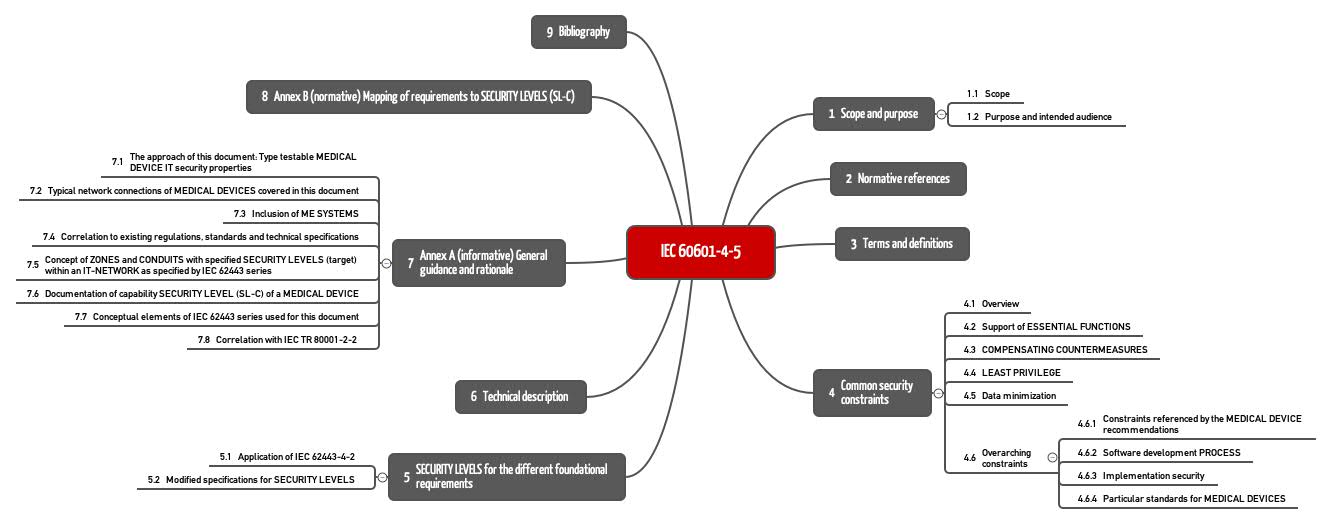
Iec 60601 4 5 The Standard For It Security Is It Also For Stand Alone Software
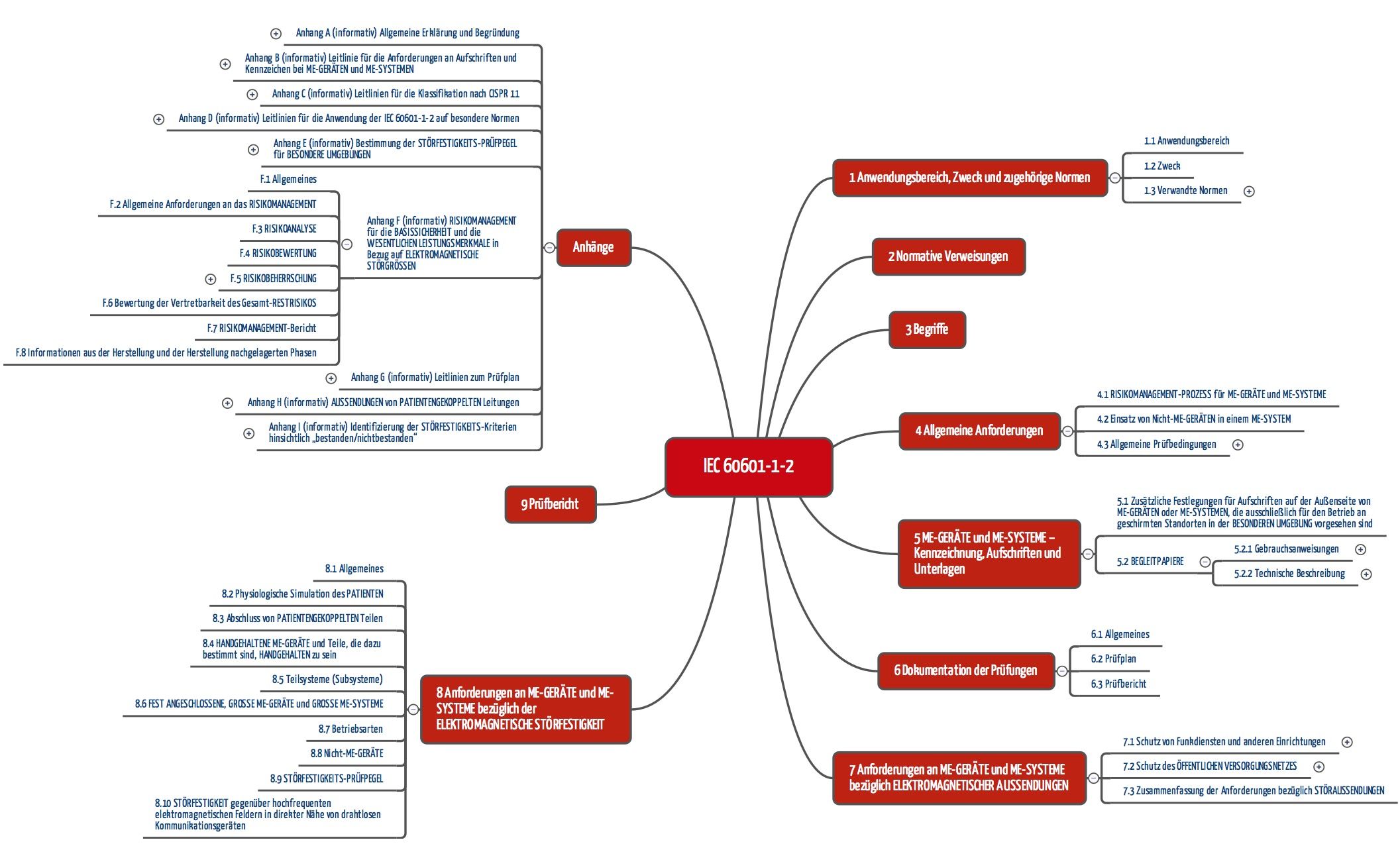
Iec 60601 1 2 Edition 4 Elektromagnetische Vertraglichkeit 2016

What Is The Iec 60601 Scope Medical Device Academy Medical Device Academy
